CBSE Class 12-science Answered
Calculate the boiling point of a solution containing 0.45g of camphor (mol. wt. 152) dissolved in 35.4g of acetone (b.p. 56.3°C); Kb per 100 gm of acetone is 17.2°C. IN THIS QUESTION WE USE THE FORMULA = 100 Kb *w2/w1M2 I want to know how we derive this formula in which 100 is in numerator and not 1000 . I know Kb is per 100 g though I am not able to derive this relation
Asked by govtsecschoolnayaganv051 | 11 Jun, 2019, 01:38: PM
Given:
Weight of solute, w = 0.45 gm
Molecular weight, M = 152
Weight of solvent, W = 35.4 gm
Molal elevation constant, Kb = 17.2 per 100 gm
We have,
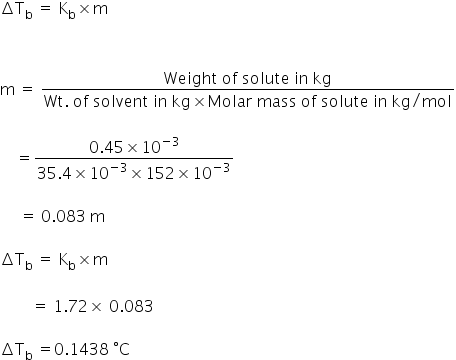
ΔTb = B.P of solution(T) - B.P of solvent( T0)
B.P of solution(T) = T0 + Tb
= 56.3 + 0.1438
B.P of solution(T) = 56.44 °C
The boiling point of solution is 56.44 °C.
Answered by Varsha | 12 Jun, 2019, 10:28: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by varinder2149 | 10 Dec, 2023, 08:21: PM
CBSE 12-science - Chemistry
Asked by vekariyaparth61 | 16 May, 2022, 04:33: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:27: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:25: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:20: PM
CBSE 12-science - Chemistry
Asked by sshashu993 | 25 Jul, 2020, 08:02: AM
CBSE 12-science - Chemistry
Asked by anukritisingh8103.bmps | 15 Jul, 2020, 05:52: PM
CBSE 12-science - Chemistry
Asked by sharmasherryal | 25 May, 2020, 09:54: AM
CBSE 12-science - Chemistry
Asked by panthpreet0221 | 06 May, 2020, 10:41: AM