CBSE Class 10 Answered
Calculate pH of a solution which contains 9.9 ml of 1 M HCl and 100 ml of 0.1 M NaOH.
Asked by acv27joy | 06 Oct, 2018, 09:21: PM
The reaction proceeds as :
NaOH + HCl → NaCl + H2O
As we know,
1 mole of HCl reacts with the 1 mole of NaOH
lets find the moles of solution,
Mole HCl in 9.9 ml of 1 M solution = 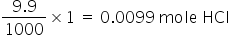
Mole NaOHin 100 ml of 0.1 M solution = 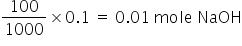
on mixing the 0.0099 mole of HCl will be neutralised = 0.01 - 0.0099 = 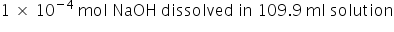
hence, the molarity of NaOH solution = 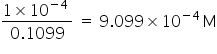
As we find the molarity lets calculate the pH of solution,
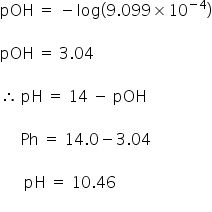
Answered by Ramandeep | 12 Oct, 2018, 12:57: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by kamalapallysudha17 | 25 Mar, 2024, 07:52: PM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 23 Nov, 2021, 02:45: AM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 23 Nov, 2021, 02:28: AM
CBSE 10 - Chemistry
Asked by psuryanshi2903 | 15 Oct, 2020, 04:31: PM
CBSE 10 - Chemistry
Asked by arindeep.singh | 12 Jul, 2020, 01:10: PM
CBSE 10 - Chemistry
Asked by niyati2600 | 04 Apr, 2020, 03:14: PM
CBSE 10 - Chemistry
Asked by aman8003singh | 20 Mar, 2020, 11:10: PM
CBSE 10 - Chemistry
Asked by aman8003singh | 20 Mar, 2020, 11:08: PM
CBSE 10 - Chemistry
Asked by jayant_abraham | 04 Mar, 2020, 09:59: PM





