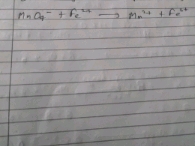CBSE Class 11-science Answered
calcium carbonate reacts with aq HCL to give CaCl2 & CO2 according to the reaction given below :
CaCo3+2HCL(aq)+CO2+H2O
what mass of CaCl2 will be formed whin 250ml of 0.76M HCL react with 1000g of CaCo3? name the limiting reagent. calculate the no. of moles of CaCl2 formed in the reaction.
Asked by shivani sharma | 28 Aug, 2012, 06:05: PM
Now, according to the definition of molarity, the no. of moles are calculated for 1000 ml or 1 litre of the solution.
1000 ml of 0.75 M HCl contains HCl = 0.75 mol
So, 25 ml of 0.75 M HCl will contain HCl = 0.75x25/1000 = 0.01875 mol
2 mol of HCl reacts with = 1 mol of CaCO3
So, 0.01875 mol of HCl will react with = 1x0.01875/2 = 0.009375 mol
Molar mass of CaCO3 = 100 g
Hence, the mass of 0.009375 mol of CaCO3 = no. of moles x molar mass
= 0.009375 x 100 = 0.9375 g
Answered by | 31 Aug, 2012, 05:32: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by VarunTYAGi9013 | 21 Oct, 2023, 07:14: PM