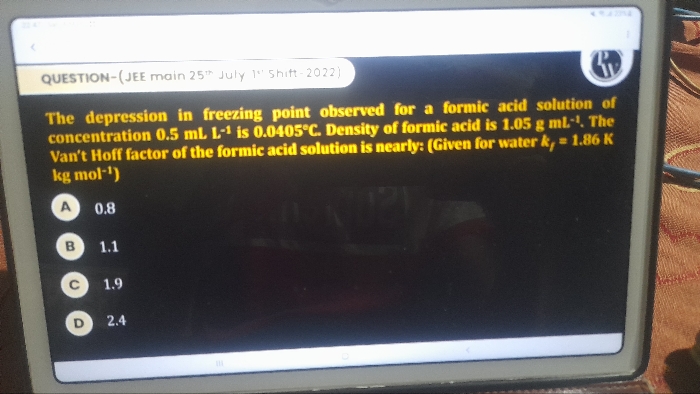
JEE Class main Answered
Benzene and toulene form an ideal solution. The vapour pressure of benzene and toulene are 75 mm and 22 mm at 20°c.If the mole fraction of benzene and toulene in vapour phase are 0.63 and 0.37 respectively. Calculate the vapour pressure of ideal mixture.
Asked by sunilpatil4411 | 06 Mar, 2019, 19:34: PM
Given:
Vapour pressure of benzene PB0 =75 mm Hg
Vapour pressure of toluene PT0 = 22 mm Hg
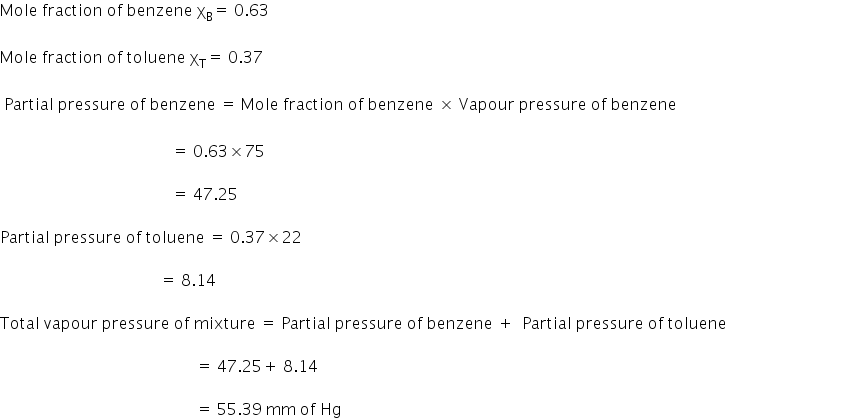
The total vapour pressure of the mixture is 55.39 mm of Hg.
Answered by Varsha | 07 Mar, 2019, 12:43: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM
JEE main - Chemistry
Asked by debnath.ankita2023 | 16 May, 2024, 13:35: PM
JEE main - Chemistry
Asked by amarnathreddyp19 | 29 Mar, 2024, 06:47: AM
JEE main - Chemistry
Asked by atharvamane801 | 14 Jan, 2024, 12:07: PM
JEE main - Chemistry
Asked by bhyogita884 | 12 Jul, 2022, 02:55: AM
JEE main - Chemistry
Asked by abdulraqeeb437 | 16 Jun, 2022, 20:38: PM
JEE main - Chemistry
Asked by akshatmi2005 | 21 May, 2021, 14:23: PM