CBSE Class 12-science Answered
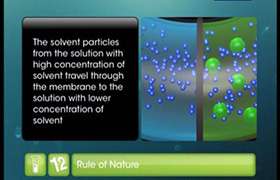
At 300 K. 18g of glucose present per litre its solution has an osmotic pressure of 4.98 bars .If the osmotic pressure of solution is 1.52 bars on the same temperature, what would be its concentration?
Asked by Topperlearning User | 20 Jun, 2016, 03:42: PM
For solution A p V= n RT
4.98 x 1L = 18/180 x R x T
For solution B p V= n RT
1.52 x 1L = n x R x T
1.52 x 1L/ n = 4.98 x 1L / 0.1
1.52 / n = 4.98 / 0.1
n = 1.52 x 0.1 / 4.98 = 0.035moles
c= 0.035 mol L-1
Answered by | 20 Jun, 2016, 05:42: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by rashmij34 | 27 Feb, 2024, 04:42: PM
CBSE 12-science - Chemistry
Asked by premkhare2006 | 24 Jan, 2024, 09:50: AM
CBSE 12-science - Chemistry
Asked by kaushikmisty07 | 31 Dec, 2023, 11:42: AM
CBSE 12-science - Chemistry
Asked by KRISHPATEL.soc | 21 Jun, 2021, 05:58: PM
CBSE 12-science - Chemistry
Asked by dhrubajyoti.das | 09 May, 2021, 09:54: PM
CBSE 12-science - Chemistry
Asked by tiwariaatman | 31 Jul, 2020, 05:10: PM
CBSE 12-science - Chemistry
Asked by yogendrasoni142 | 08 Jun, 2020, 05:43: PM
CBSE 12-science - Chemistry
Asked by santosh357m | 28 Apr, 2020, 09:28: AM
CBSE 12-science - Chemistry
Asked by Balbir | 27 Jul, 2019, 05:02: PM
CBSE 12-science - Chemistry
Asked by ajaysankhala051 | 04 Jun, 2019, 02:28: PM