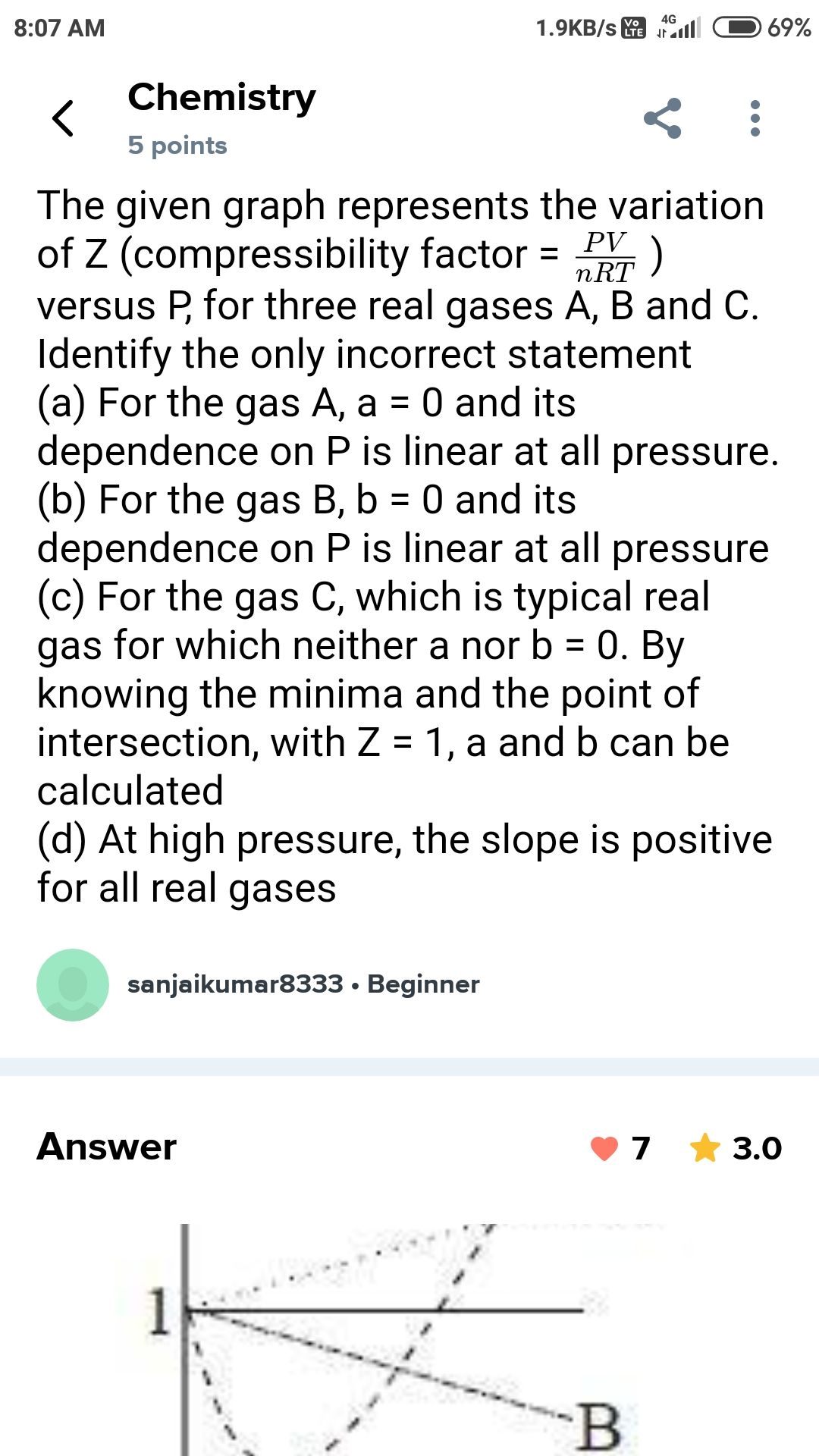
NEET Class neet Answered
Answer this question....
Asked by jhajuhi19 | 19 Aug, 2021, 08:38: PM
Dear Student,
This question is based on kinetic energy.
The average translational kinetic energy (per molecule) of any (ideal) gas is always equal to 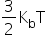 . It depends only on temperature and is independent of the nature of the gas.
. It depends only on temperature and is independent of the nature of the gas.
Since argon and chlorine both have the same temperature in the flask, the ratio of average kinetic energy (per molecule) of the two gases is 1:1.
Answered by Ravi | 20 Aug, 2021, 10:55: AM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by gopikakannan27 | 13 Jan, 2022, 05:39: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 19 Aug, 2021, 08:38: PM
NEET neet - Chemistry
Asked by kowsalyamouli | 06 Oct, 2020, 04:08: PM
NEET neet - Chemistry
Asked by nssharma001969 | 03 Jun, 2020, 01:54: AM
NEET neet - Chemistry
Asked by prakriti12oct | 25 May, 2020, 12:15: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 10 May, 2020, 08:24: AM
NEET neet - Chemistry
Asked by anushka.kulkarni02022002 | 19 Mar, 2020, 09:31: AM
NEET neet - Chemistry
Asked by Prashant DIGHE | 03 Mar, 2020, 10:09: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 03 Mar, 2020, 10:06: PM