CBSE Class 10 Answered
and water should come.
CH3COOH+CH3CH2OH -------- CH3COOC2H5+H20
Asked by K.prawin | 11 Mar, 2019, 06:46: PM
In esterification reaction, the carboxylic acid and alcohols react in the presence of concentrated sulphuric acid and formed fruity sweet smelling compounds called esters.
Concentrated H2SO4 acts as a catalyst and a dehydrating agent i.e., it removes water molecule.
The reaction: 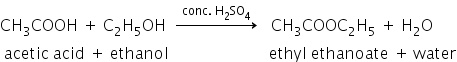
Answered by Ramandeep | 11 Mar, 2019, 07:19: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by sneh | 27 Mar, 2020, 10:11: AM
CBSE 10 - Chemistry
Asked by pranjaliinamdar2004 | 29 Feb, 2020, 07:20: PM
CBSE 10 - Chemistry
Asked by sweetykhatri99254 | 27 Feb, 2020, 03:40: PM
CBSE 10 - Chemistry
Asked by priyanshiishu | 30 Jan, 2020, 10:39: AM
CBSE 10 - Chemistry
Asked by kamalnayansingh7 | 13 Jan, 2020, 08:35: AM
CBSE 10 - Chemistry
Asked by Deepak | 22 Dec, 2019, 11:20: PM
CBSE 10 - Chemistry
Asked by ritikraghuwanshi6986 | 16 Dec, 2019, 08:42: PM
CBSE 10 - Chemistry
Asked by vedantsagrawal23 | 05 Dec, 2019, 08:34: AM
CBSE 10 - Chemistry
Asked by aryasaxena2003 | 25 Jul, 2019, 05:35: PM
CBSE 10 - Chemistry
Asked by rushabhjain.avv | 21 Mar, 2019, 10:07: PM