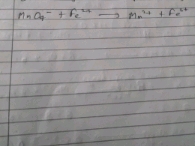CBSE Class 11-science Answered
An organic compound on analysis gave the following percentage composition:
C=57.8% ; H=3.6% and the rest is oxygen. The vapour density of the compound was found to be 83. Find the molecular formula of the compound.
Asked by Prachi Panwar | 21 May, 2013, 06:42: PM
|
Element |
% amount |
Relative number of atoms |
Ratio of number of atoms |
Simplest ratio |
|
C |
57.8 |
(57.8/12) = 4.82 |
(4.82/2.41) = 2 |
2x2 = 4 |
|
H |
3.6 |
(3.6/1) = 3.6 |
(3.6/2.41) = 1.5 |
1.5x2 = 3 |
|
O |
(100-57.8-3.6) = 38.6 |
(38.6/16) = 2.41 |
(2.41/2.41) = 1 |
1x2=2 |
So, we can deduce the empirical formula of the organic compound = C4H3O2.
The empirical Formula mass = 4x12 + 3x1 + 2x16 = 83 u.
The molecular mass of compound can be calculated as:
Molecular mass = 2 x Vapour Density = 2 x 83 = 166 u.
n = Molecular mass / Empirical Formula Mass = 166/83 = 2.
Molecular Formula of the compound = n(Empirical Formula) = 2(C4H3O2) = C8H6O4.
Answered by | 21 May, 2013, 09:54: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by VarunTYAGi9013 | 21 Oct, 2023, 07:14: PM