CBSE Class 11-science Answered
an organic compound an analysis : c=65%, h=3.5% n = 9.6% and o= 21.9% 0.3675 gm of
the

Asked by blpradhanpradhan94137 | 01 Jun, 2020, 10:46: AM
Given:
C = 65%
H=3.5%
N=9.6%
O=21.9%
Mass of organic compound is 0.3675gm
| Element | % | No. of moles | simple ratio |
| C | 65 | 65/12= 5.42 | 5.42/0.68=7.97=8 |
| H | 3.5 | 3.5/1=3.5 | 3.5/0.68= 5.1=5 |
| N | 9.6 | 9.6/14=0.68 | 0.68/0.68=1 |
| O | 21.9 | 21.9/16=1.37 | 1.37/0.68= 2.01 =2 |
Empirical formula = C8H5NO2, mass of emperical formula = (8× 12)+(1×5)+(1×14)+(16×2) =168+5+14+32=219
Now, 53cc of compound = 0.3675gm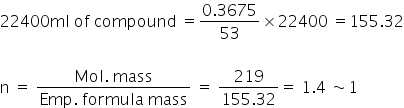
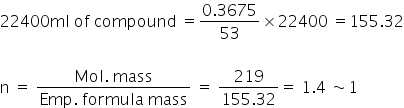
Therefore molecular formula= C8H5NO2
Answered by Ramandeep | 08 Jun, 2020, 01:19: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by hcnainwal | 15 Jun, 2023, 10:39: AM
CBSE 11-science - Chemistry
Asked by Jprmumal29 | 18 Dec, 2022, 09:48: PM
CBSE 11-science - Chemistry
Asked by mallikarjunasangi28 | 22 Jul, 2022, 07:57: PM
CBSE 11-science - Chemistry
Asked by vedwatisharma79 | 10 Jun, 2022, 05:27: PM
CBSE 11-science - Chemistry
Asked by thathvakunjusree | 10 Dec, 2021, 06:46: AM
CBSE 11-science - Chemistry
Asked by udheshraddha2004 | 28 Oct, 2021, 09:37: PM
CBSE 11-science - Chemistry
Asked by arunparewa2000 | 27 Oct, 2021, 06:59: PM
CBSE 11-science - Chemistry
Asked by arttameher038 | 23 Aug, 2021, 07:06: AM







