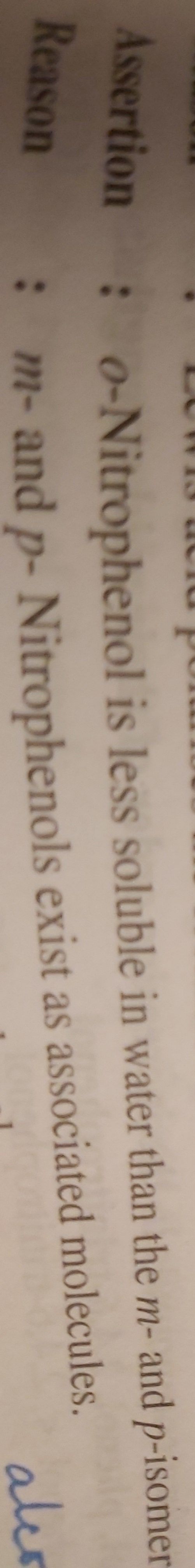CBSE Class 12-science Answered
account for the following
Asked by | 28 Feb, 2008, 10:38: PM
o-nitrophenol is more acidic then p-nitrophenol
the distance of the electron withdrawing group is more in para-nitrophenol so the release of a proton will be less as compared to o-nitro phenol.
Answered by | 20 Dec, 2017, 04:44: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kavitabawane190 | 08 Mar, 2024, 05:24: PM
CBSE 12-science - Chemistry
Asked by rp0055293 | 07 Feb, 2024, 08:28: AM
CBSE 12-science - Chemistry
Asked by vipulverma | 14 Feb, 2022, 04:44: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 23 Jun, 2021, 08:02: PM
CBSE 12-science - Chemistry
Asked by Rg598555 | 30 Oct, 2019, 10:35: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 04:13: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Mar, 2014, 03:43: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 03:12: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 03:17: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Mar, 2014, 04:54: PM