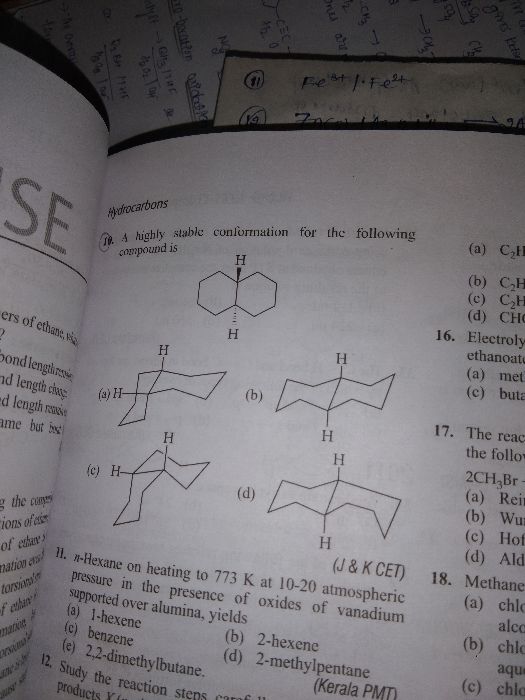
CBSE Class 11-science Answered
A:tetracyanomethane ; B:carbondioxide
C:benzene ; D: 1,3-buta-di-ene
Ratio of sigma and pi bonds is in order:
options:
(a)A=B<C<D
(b)A=B<D<C
(c)A=B=C=D
(d)C<D<A<B
please explain the correct answer.
Asked by Yash Sahu | 09 Apr, 2014, 03:05: PM
Option (b) A=B<D<C is correct.
In tetracyanomethane, there are total 8 sigma and 8 pi bonds. Hence the ration of sigma to pi is 1:1.
In carbon dioxide, there are 2 sigma and 2 pi bonds, hence again the raio is 1:1.
In benzene, the total sigma bonds are 12 and pi bonds are 3, hence the ratio is 4:1
In 1,3-butadiene, there are c3 sigma bonds and 2 pi bonds , hence the ration is 3:2 or we can say, 1.5:1.
Thus the least ratio is 1:1 of both A and B compounds, then 1.5:1 of D and finally the highest ratio is 4:1 of C.
Answered by | 09 Apr, 2014, 05:27: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by kirithshiv | 24 Feb, 2024, 12:12: PM
CBSE 11-science - Chemistry
Asked by shivanij4734 | 16 Dec, 2023, 08:30: PM
CBSE 11-science - Chemistry
Asked by maibamjohnny89 | 15 Jan, 2022, 09:38: PM
CBSE 11-science - Chemistry
Asked by archu312004 | 07 Feb, 2021, 10:21: PM
CBSE 11-science - Chemistry
Asked by dubeyanubhav65 | 18 Jan, 2021, 09:52: PM
CBSE 11-science - Chemistry
Asked by shahsaqlain107 | 30 Nov, 2020, 01:10: PM
CBSE 11-science - Chemistry
Asked by ghastipratiksha | 11 Jul, 2020, 08:14: PM
CBSE 11-science - Chemistry
Asked by guptaserendri | 01 Jul, 2020, 03:58: PM
CBSE 11-science - Chemistry
Asked by Manpreetsingh669933 | 13 Apr, 2020, 01:39: PM
CBSE 11-science - Chemistry
Asked by bittutiwary1234 | 23 Feb, 2020, 12:15: AM