CBSE Class 11-science Answered
a solution contains 100g of naoh and 100 g of h2so4 . the solution is acidic, basic, or neutral?
Asked by Harshit Agarwal | 24 Aug, 2014, 12:05: PM
No. of moles of NaOH = 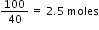
No. of moles of H2SO4 = 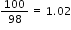 moles
moles
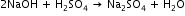
From above reaction it is clear that 1 mole of H2SO4 require 2 moles of NaOH for neutralisation.
So 1.02 x 2 = 2.04 moles of NaOH will be required for neutralisation.
2.5 - 2.04 = 0.46 moles of NaOH are extra.
So the resulting solution will be basic.
Answered by Arvind Diwale | 25 Aug, 2014, 10:51: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by hcnainwal | 15 Jun, 2023, 10:39: AM
CBSE 11-science - Chemistry
Asked by Jprmumal29 | 18 Dec, 2022, 09:48: PM
CBSE 11-science - Chemistry
Asked by mallikarjunasangi28 | 22 Jul, 2022, 07:57: PM
CBSE 11-science - Chemistry
Asked by vedwatisharma79 | 10 Jun, 2022, 05:27: PM
CBSE 11-science - Chemistry
Asked by thathvakunjusree | 10 Dec, 2021, 06:46: AM
CBSE 11-science - Chemistry
Asked by udheshraddha2004 | 28 Oct, 2021, 09:37: PM
CBSE 11-science - Chemistry
Asked by arunparewa2000 | 27 Oct, 2021, 06:59: PM
CBSE 11-science - Chemistry
Asked by arttameher038 | 23 Aug, 2021, 07:06: AM







