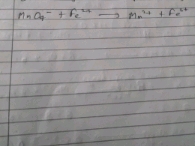CBSE Class 11-science Answered
a sample of CaCo3 is 50% pure on heating 1.12L of Co2(al STP) is obtained.residue left is- a)7.8 b)3.8 c)2.8 d)8.9
Asked by | 02 Dec, 2012, 08:28: PM
CaCO3 decomposes as following reaction:
CaCO3 ? CaO + CO2
According to the reaction, 1 mol CaCO3 produces 1 mol CO2
At STP 1mol CO2 = 22.4L
We have 1.12L = 1.12/22.4 = 0.05 mol CO2
Therefore we had 0.05 mol CaCO3 to start.
Molar mass CaCO3 = 100g/mol
Now, we had 0.05mol = 0.05*100 = 5g CaCO3
we started with 5g CaCO3 and 5g Impurity
Molar mass CaO = 56g/mol
Mass of CaO remaining = 5* 56/100 = 2.8g
Total mass residue = 5+2.8 = 7.8g
Choice 1 is correct.
We have 1.12L = 1.12/22.4 = 0.05 mol CO2
Therefore we had 0.05 mol CaCO3 to start.
Molar mass CaCO3 = 100g/mol
Now, we had 0.05mol = 0.05*100 = 5g CaCO3
we started with 5g CaCO3 and 5g Impurity
Molar mass CaO = 56g/mol
Mass of CaO remaining = 5* 56/100 = 2.8g
Total mass residue = 5+2.8 = 7.8g
Choice 1 is correct.
Answered by | 14 Dec, 2012, 07:18: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by VarunTYAGi9013 | 21 Oct, 2023, 07:14: PM