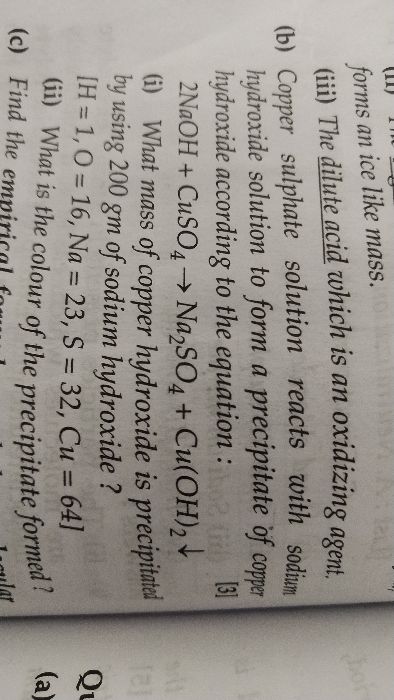ICSE Class 10 Answered
A mixture of hydrogen and chlorine occupying 36 cm3 was exploded. On shaking it with water, 4cm3 of hydrogen was left behind. Find the composition of the mixture.
Asked by aashimegh | 04 Sep, 2019, 08:53: AM
The chemical equation is given by:

1 mole Hydrogen H2 reacts with 1 mole chlorine Cl2 to form 2 moles of HCl.
Therefore, 1 vol H2 reacts with 1 vol Cl2 and forms 2 vol HCl.
H2 and Cl2 reacts in equal volumes.
As 4 cm3 H2 is left after the completion of reaction, the volume of gases reacting must be 16 cm3 H2 and 16 cm3 Cl2
Hence, the composition of mixture is:
16 + 4 = 20 cm3 of H2 and 16 cm3 of Cl2.
Answered by Varsha | 04 Sep, 2019, 11:42: AM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by jrvedant208 | 05 Feb, 2024, 10:37: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 10 Jul, 2022, 10:13: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 25 Jun, 2022, 10:24: PM
ICSE 10 - Chemistry
Asked by palshivom72 | 14 Jul, 2020, 07:56: PM
ICSE 10 - Chemistry
Asked by jhabijay01 | 27 May, 2020, 12:20: PM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:53: AM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:37: AM
ICSE 10 - Chemistry
Asked by aashimegh | 28 Aug, 2019, 05:25: PM