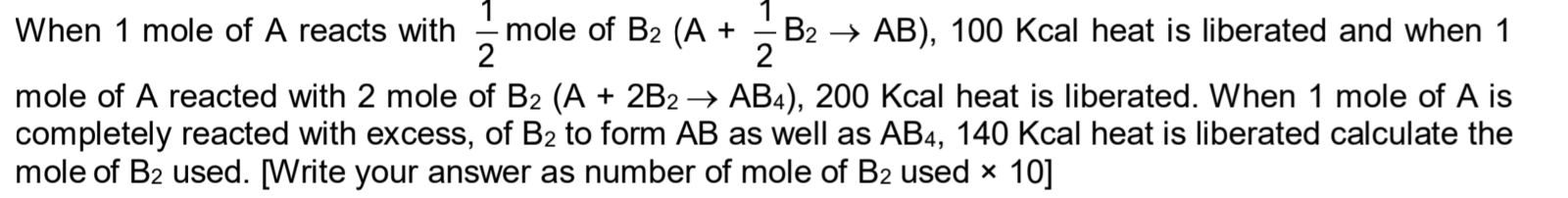CBSE Class 11-science Answered
A mixture of FeO and Fe3O4 when heated in air to constant weight gains 5% in its weight. The % of FeO in the sample is
Asked by Anil | 19 May, 2017, 10:28: PM
Let us consider weight of FeO & Fe3O4 be 'a' & 'b' gm respectively.
Consider the following reactions.
2 FeO + 1/2 O2 ------> Fe2O3
Fe3O4 + 1/2 O2 ------> 3Fe2O3
Molar mass of FeO =144 g and that of Fe2O3 is 160 gms.
Thus 144 g FeO gives 160 g Fe2O3
'a' g FeO will give 160 x a/144 gm Fe2O3 --------------(1)
Similarly, weight of Fe2O3 formed by ‘b’gm Fe3O4 = 160 x 3b/464 --------(2)
Assuming total weight i.e. (a+b) = 100. -------- (3)
As there is an increase of 5 % we get, from eqn. 1,2 & 3 .
(160 x a /144) +160 x 3b/464 = 105
solving we get,
a= 21.06 & b = 78.94
Thus % of FeO = 21.06 % & that of Fe3O4 = 78.94 %
Answered by Vaibhav Chavan | 20 May, 2017, 06:29: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by drhimasingh | 22 May, 2020, 11:39: AM
CBSE 11-science - Chemistry
Asked by nareshrajpurohit43109 | 22 May, 2020, 11:18: AM
CBSE 11-science - Chemistry
Asked by d6knx7qmw1 | 15 May, 2020, 10:37: PM
CBSE 11-science - Chemistry
Asked by sahadipa1975 | 02 May, 2020, 08:53: AM
CBSE 11-science - Chemistry
Asked by abhishek19362771 | 08 Apr, 2020, 03:48: PM
CBSE 11-science - Chemistry
Asked by anilsolanki2060 | 22 Feb, 2020, 10:12: AM
CBSE 11-science - Chemistry
Asked by pujakurmi22 | 11 Nov, 2019, 10:59: PM
CBSE 11-science - Chemistry
Asked by jkatwara | 14 Oct, 2019, 12:21: PM
CBSE 11-science - Chemistry
Asked by vikas.kochhar6 | 30 Aug, 2019, 03:58: PM
CBSE 11-science - Chemistry
Asked by pb_ckt | 19 May, 2019, 11:56: PM