JEE Class main Answered
a hydrocarbon on combustion gives CO2 and H2O in a volume ratio of 2 ratio 1 under single condition temperature and pressure what is the empirical formula of the hydrocarbon
Asked by nitindeephuda | 20 May, 2021, 08:32: AM
As we know that the balanced chemical reaction for the combustion is-
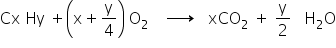 Now according to the question,
Now according to the question,
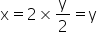 Thus the empirical formula of hydrocarbon is CH.
Thus the empirical formula of hydrocarbon is CH.
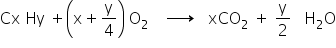
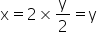
Answered by Ramandeep | 20 May, 2021, 18:22: PM
Application Videos
Concept Videos
JEE main - Chemistry
Asked by neerajavuppala1983 | 23 Jul, 2024, 22:49: PM
JEE main - Chemistry
Asked by tanniruv133 | 03 Jul, 2024, 18:50: PM
JEE main - Chemistry
Asked by heyyyyy | 12 Jun, 2024, 19:18: PM
JEE main - Chemistry
Asked by hv5594265 | 12 Jun, 2024, 11:59: AM
JEE main - Chemistry
Asked by rupalibhange1987 | 11 Jun, 2024, 20:00: PM
JEE main - Chemistry
Asked by gajju8493 | 11 Jun, 2024, 15:09: PM
JEE main - Chemistry
Asked by chakrabortymithu041 | 29 May, 2024, 18:45: PM













