CBSE Class 10 Answered
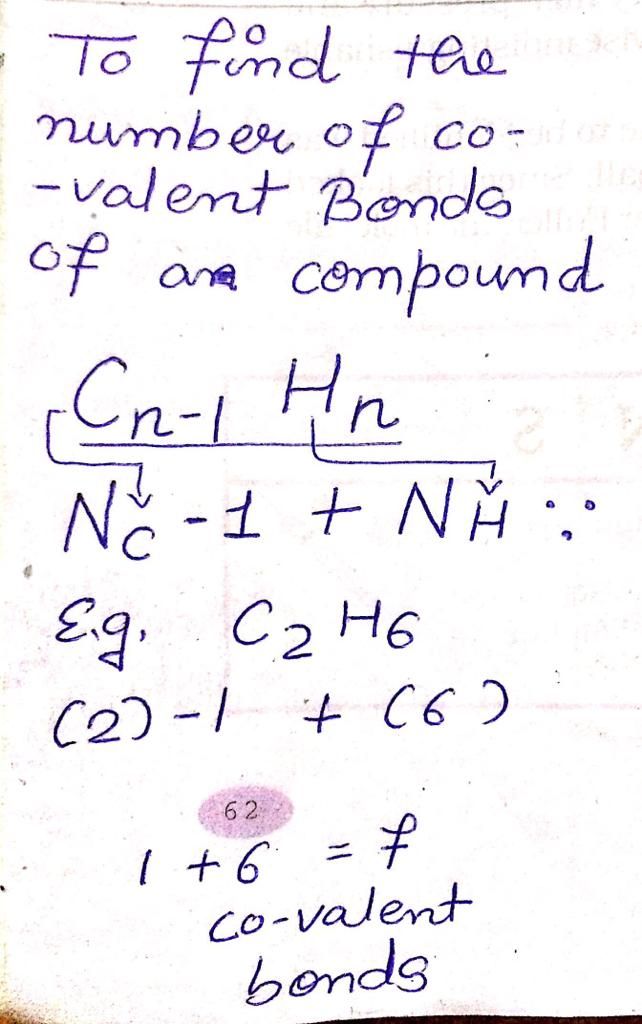
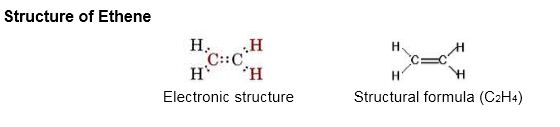
A compound 'X' is C2H4. Draw its electron dot structure.Will it dissolve in water or not? Will it conduct electricity in aqueous solution? Will it have high melting or low melting point?
Asked by Omkar | 02 Jan, 2018, 04:41: PM
Compound X is Ethene (C2H4).
Electron dot structure of Ethene:
- Sparingly soluble in water but highly soluble in organic solvents.
- It does not conduct electricity.
- The boiling point is −102°C, and melting point is −169°C.
Answered by Ramandeep | 03 Jan, 2018, 10:08: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by tialempongen177 | 09 Sep, 2020, 11:44: PM
CBSE 10 - Chemistry
Asked by seeni2005 | 05 Jul, 2020, 09:53: PM
CBSE 10 - Chemistry
Asked by subbukum | 04 Feb, 2020, 11:46: AM
CBSE 10 - Chemistry
Asked by navjotsinghdadwal | 01 Dec, 2019, 09:57: PM
CBSE 10 - Chemistry
Asked by 9886761796hmh | 17 Oct, 2019, 08:10: PM
CBSE 10 - Chemistry
Asked by ashishaman25082004 | 15 Sep, 2019, 10:01: PM
CBSE 10 - Chemistry
Asked by dr.sudhiguptajdmd74 | 23 Jul, 2019, 09:18: AM
CBSE 10 - Chemistry
Asked by labheshvaidya | 19 Apr, 2019, 03:38: PM
CBSE 10 - Chemistry
Asked by krishdabhoya2003 | 21 Feb, 2019, 05:52: PM
CBSE 10 - Chemistry
Asked by amitpass78 | 09 Jan, 2019, 10:35: PM