CBSE Class 11-science Answered
A coating of cobalt that is 0.005cm thick is deposited on a plate that is 0.5cm^2 in total area. How many atoms of cobalt are deposited in a plate? (given density of cobalt = 8.9g cm ^-3 , atomic mass of Co = 59 amu)
Asked by Diya | 26 Apr, 2018, 01:48: AM
Given:
Thickness= 0.005 cm
Area =0.5 cm2
Density of Co = 8.9gcm-3
Molar mass of Co = 59
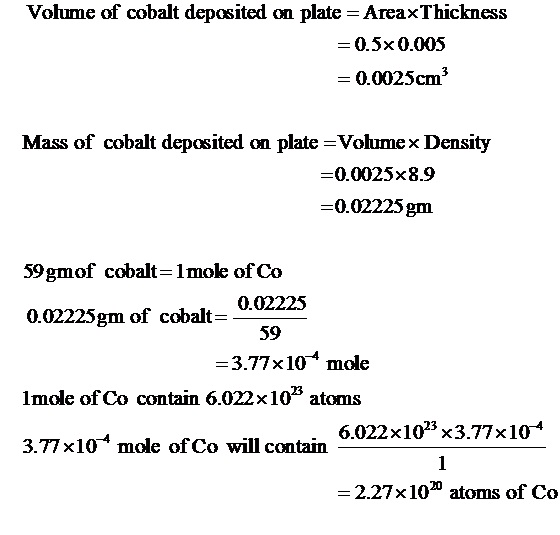
Therefore, 2.27×1020 atoms of cobalt are deposited on a plate.
Answered by Varsha | 26 Apr, 2018, 12:36: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by bablipanwar893 | 01 Jul, 2023, 12:25: PM
CBSE 11-science - Chemistry
Asked by saijagdale9 | 19 Jun, 2023, 02:34: PM
CBSE 11-science - Chemistry
Asked by kdimple765 | 17 Jul, 2022, 01:24: PM
CBSE 11-science - Chemistry
Asked by alfirozislam900 | 03 Jul, 2022, 01:24: PM
CBSE 11-science - Chemistry
Asked by alfirozislam900 | 03 Jul, 2022, 01:23: PM




