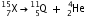ICSE Class 10 Answered
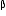 -particle, (iii) one
-particle, (iii) one 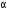 - particle.
Write the symbol of the new nucleus in each case and express each change by a reaction.
- particle.
Write the symbol of the new nucleus in each case and express each change by a reaction.(a) Mass number = number of protons + number of neutrons and atomic number = number of protons.
Therefore, number of neutrons = mass number - atomic number = 15 - 7 = 8.
(b) Element X can be written as 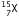 .
.
(c) (i) After the loss of 1 proton, the mass number and atomic number of the nucleus 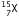 will decrease by 1. The new nucleus will be
will decrease by 1. The new nucleus will be 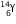 (say). The change can be written as :
(say). The change can be written as :
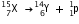
(ii) After the loss of one 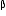 -particle, the mass number will remain the same but the atomic number will increase by 1. The element
-particle, the mass number will remain the same but the atomic number will increase by 1. The element 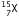 changes to
changes to 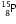 (say) as follows :
(say) as follows :
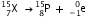
(iii) After the loss of one 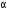 -particle, the mass number decreases by 4 and the atomic number decreases by 2. The element
-particle, the mass number decreases by 4 and the atomic number decreases by 2. The element 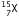 changes to
changes to 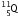 (say) as follows :
(say) as follows :