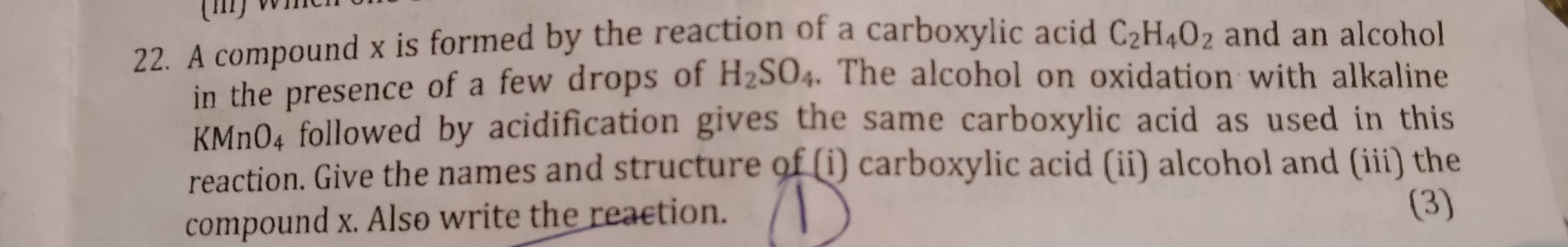CBSE Class 10 Answered
A carbon compound ‘P’ on heating with excess conc.
H2
SO4
forms another carbon compound ‘Q’ which on
addition of hydrogen in the presence of nickel catalyst
forms a saturated carbon compound ‘R’. One molecule
of ‘R’ on combustion forms two molecules of carbon
dioxide and three molecules of water. Identify P, Q
and R and write chemical equations for the reactions
involved.
Asked by priyanshiishu | 30 Jan, 2020, 10:39: AM
When P reacts with Q.

Answered by Ravi | 31 Jan, 2020, 03:43: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by sneh | 27 Mar, 2020, 10:11: AM
CBSE 10 - Chemistry
Asked by pranjaliinamdar2004 | 29 Feb, 2020, 07:20: PM
CBSE 10 - Chemistry
Asked by sweetykhatri99254 | 27 Feb, 2020, 03:40: PM
CBSE 10 - Chemistry
Asked by priyanshiishu | 30 Jan, 2020, 10:39: AM
CBSE 10 - Chemistry
Asked by kamalnayansingh7 | 13 Jan, 2020, 08:35: AM
CBSE 10 - Chemistry
Asked by Deepak | 22 Dec, 2019, 11:20: PM
CBSE 10 - Chemistry
Asked by ritikraghuwanshi6986 | 16 Dec, 2019, 08:42: PM
CBSE 10 - Chemistry
Asked by vedantsagrawal23 | 05 Dec, 2019, 08:34: AM
CBSE 10 - Chemistry
Asked by aryasaxena2003 | 25 Jul, 2019, 05:35: PM
CBSE 10 - Chemistry
Asked by rushabhjain.avv | 21 Mar, 2019, 10:07: PM