CBSE Class 12-science Answered
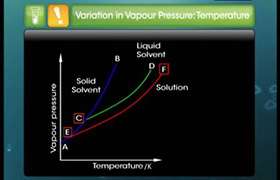
A and B liquids on mixing produved warm solution.which type of deviation is there and why?
Asked by | 29 Feb, 2008, 08:19: AM
Solution becomes hot means heat is given out and it is a exothermic reaction. So  H is negative or the intermolecular attraction of reactant A-A&B-B is> A-B interaction in solution. it will show positive deviation.
H is negative or the intermolecular attraction of reactant A-A&B-B is> A-B interaction in solution. it will show positive deviation.
Answered by | 20 Dec, 2017, 04:29: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by varinder2149 | 10 Dec, 2023, 08:21: PM
CBSE 12-science - Chemistry
Asked by vekariyaparth61 | 16 May, 2022, 04:33: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:27: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:25: PM
CBSE 12-science - Chemistry
Asked by goyalpavitarta | 03 May, 2021, 01:20: PM
CBSE 12-science - Chemistry
Asked by sshashu993 | 25 Jul, 2020, 08:02: AM
CBSE 12-science - Chemistry
Asked by anukritisingh8103.bmps | 15 Jul, 2020, 05:52: PM
CBSE 12-science - Chemistry
Asked by sharmasherryal | 25 May, 2020, 09:54: AM
CBSE 12-science - Chemistry
Asked by panthpreet0221 | 06 May, 2020, 10:41: AM