CBSE Class 11-science Answered
50ml of 0.10M HCL is mixed with 50ml of 0.10M NAOH.The solution of the temperature rises by 276K.Calculate the enthalpy of neutralization per mole of HCL.
1)-2.5*100 KJ
2)-1.3*100KJ
3)-8.4*10KJ
4)-6.3*10KJ
Asked by TVISHA Bhatt | 17 Aug, 2014, 03:32: PM
The equation for the reaction is
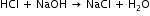
1 mole of acid reacts with 1 mole of alkali to form 1 mole of water.
Number of moles of acid used = 0.05 x 0.1 = 0.005
Number of moles of alkali used = 0.05 x 0.1 = 0.005
So number of moles of water formed = 0.005
Use formula,
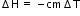
c = 4.18 kJ / kg °C, m = mass of mixed solution = 50 + 50 = 100 /1000 = 0.1 kg, = 3 °C
= 3 °C
 = 3 °C
= 3 °Cso, 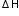 = - 4.18 x 0.1 x 3 = 1.254 kJ
= - 4.18 x 0.1 x 3 = 1.254 kJ
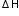 = - 4.18 x 0.1 x 3 = 1.254 kJ
= - 4.18 x 0.1 x 3 = 1.254 kJ0.005 moles  1.254 kJ
1.254 kJ
 1.254 kJ
1.254 kJ1 mole 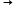
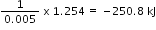 = - 2.5 x 102 kJ
= - 2.5 x 102 kJ
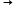
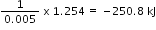 = - 2.5 x 102 kJ
= - 2.5 x 102 kJ
Answered by Arvind Diwale | 19 Aug, 2014, 11:24: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by advssdrall | 11 Jan, 2022, 07:44: PM
CBSE 11-science - Chemistry
Asked by adityasolanki7773 | 22 Oct, 2020, 03:40: PM
CBSE 11-science - Chemistry
Asked by pranavisrihari | 08 Sep, 2020, 05:24: PM
CBSE 11-science - Chemistry
Asked by varakalasuchi3 | 28 Mar, 2020, 04:47: PM
CBSE 11-science - Chemistry
Asked by patra04011965 | 09 Nov, 2019, 12:18: PM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 27 Sep, 2019, 01:43: AM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 26 Sep, 2019, 01:40: AM
CBSE 11-science - Chemistry
Asked by sayantan.chem2 | 06 Aug, 2019, 05:07: PM
CBSE 11-science - Chemistry
Asked by lovemaan5500 | 21 Jan, 2019, 06:37: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 12:24: AM