CBSE Class 9 Answered
3.6gram sample on combustion, gave 3.3 grams of CO2. The percentage of carbon in the sample is:
Asked by vikasg13.hardware | 11 Nov, 2017, 01:51: PM
% C= 12/44wt of CO2/wt* 100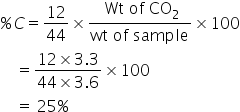
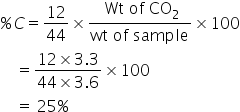
=12/44*3.3/3.6*100
=25
Answered by Varsha | 12 Nov, 2017, 10:28: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by Niharikadhamija13 | 25 Aug, 2020, 05:17: PM
CBSE 9 - Chemistry
Asked by haritchahar | 25 Jul, 2020, 11:55: AM
CBSE 9 - Chemistry
Asked by yanagauswami00.tl | 17 Apr, 2020, 10:44: AM
CBSE 9 - Chemistry
Asked by harshilmodi74.tl | 16 Apr, 2020, 10:39: AM
CBSE 9 - Chemistry
Asked by harshilmodi74.tl | 16 Apr, 2020, 10:35: AM
CBSE 9 - Chemistry
Asked by rjinaaishu007 | 10 Feb, 2020, 07:05: PM
CBSE 9 - Chemistry
Asked by prakash.sanyasi | 09 Feb, 2020, 10:59: PM
CBSE 9 - Chemistry
Asked by kumaruditanshu27 | 21 Oct, 2019, 06:16: PM
CBSE 9 - Chemistry
Asked by guptarushil6 | 15 Oct, 2019, 10:41: PM
CBSE 9 - Chemistry
Asked by lopamudrabasak1996 | 11 Aug, 2019, 12:51: AM