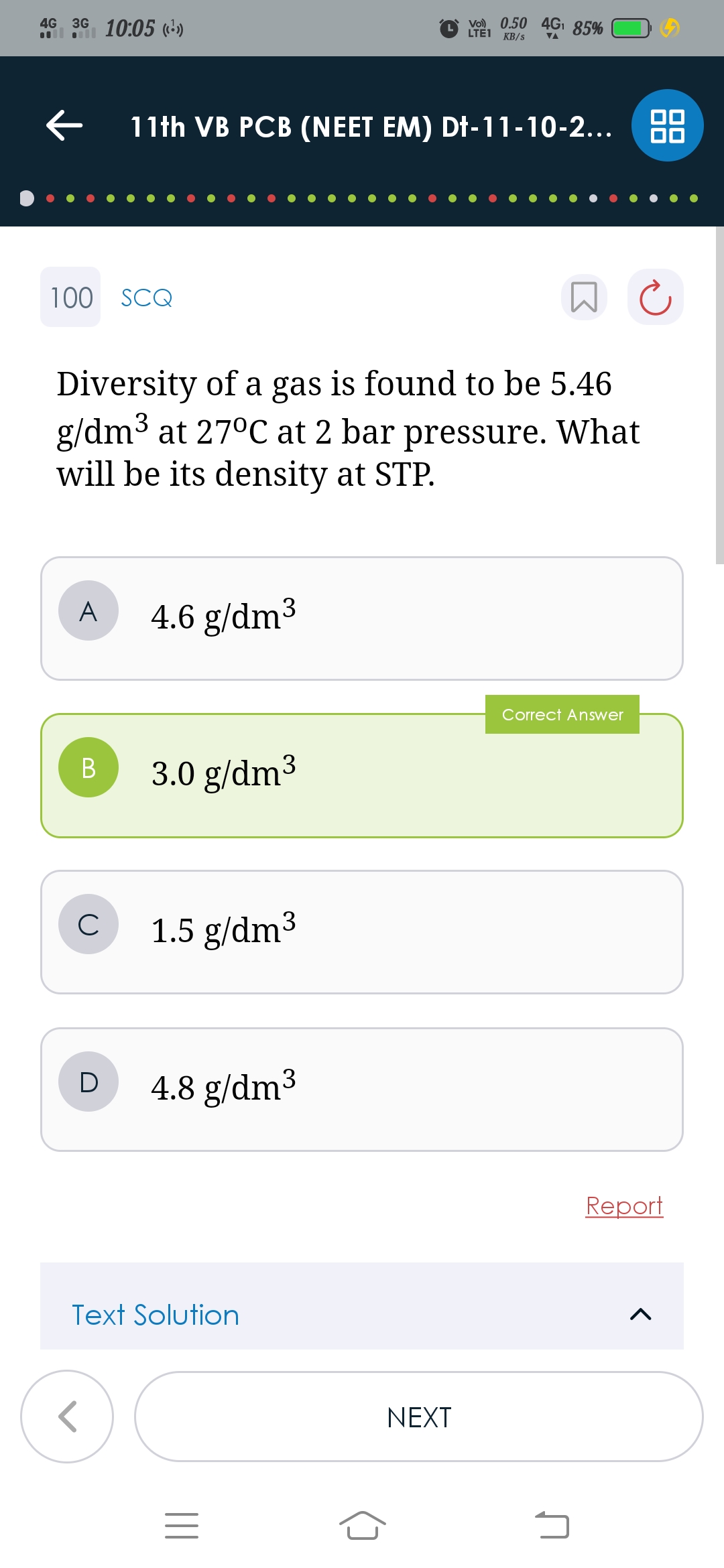
CBSE Class 11-science Answered
3 moles of an ideal gas occupies 100L under certain conditions .1.5 moles of the gas is removed and the temperature of the gas is doubled keeping the pressure constant . what is the new volume of the gas?
(A)50L
(B)200L
(C)400L
(D)100L
Asked by Yash Sahu | 16 Apr, 2014, 06:45: PM
PV = nRT
P1V1= n1RT1
P2V2 = n2RT2
P1= P2= P
P = n1RT1 / V1 and P = n2RT2 / V2
T2= 2 T1, V1= 100 L, n1= 3 moles and n2 = 1.5 moles
n1T1 / V1 = n2 x 2T1 / V2
3/100 = 1.5 x 2 / V2
V2 = 1.5 x 2 x 100 / 3
V2 = 100 L
The new volume is 100 L.
Answered by | 17 Apr, 2014, 10:52: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by rhythmdraco42 | 22 Apr, 2024, 10:43: PM
CBSE 11-science - Chemistry
Asked by vishalrolaniya2005 | 20 Sep, 2023, 08:58: PM
CBSE 11-science - Chemistry
Asked by khansuhana410 | 22 Dec, 2022, 11:48: AM
CBSE 11-science - Chemistry
Asked by amanpatel95698 | 02 Mar, 2022, 12:09: AM
CBSE 11-science - Chemistry
Asked by neerudhawanbpsmv | 28 Jun, 2021, 09:22: AM
CBSE 11-science - Chemistry
Asked by pushpakumari291279 | 31 Dec, 2020, 02:02: PM
CBSE 11-science - Chemistry
Asked by aryanvankar88 | 11 Oct, 2020, 10:07: PM
CBSE 11-science - Chemistry
Asked by nk581346 | 07 Sep, 2020, 11:08: AM
CBSE 11-science - Chemistry
Asked by swatipuspapatel | 10 Jun, 2020, 06:19: PM
CBSE 11-science - Chemistry
Asked by pratikshyadashrkl | 12 Apr, 2020, 06:47: PM