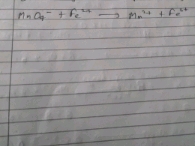CBSE Class 11-science Answered
3 and 4 are subscripts. Please explain the method.
Asked by Sayoni Maiti | 18 Apr, 2014, 07:20: PM
Given:
Two metallic oxides contain 27.6% and 30% oxygen respectively.
Ratio of metal and oxygen in first oxide, M3O4 = 72.4 : 27.6
Ratio of metal and oxygen in second oxide = 70 : 30
Let molecular mass of metal = M
Therefore, the percentage by weight of the metal in the oxide
Now,
Moles of metal in second oxide = 70 / 56 = 1.25
Moles of oxygen in second oxide = 30 / 16 = 1.875
Ration of moles of metal and oxygen = 1.25 : 1.875
= 1 : 1.5
= 2 : 3
Hence, formula of the second oxide = M2O3
Answered by | 21 Apr, 2014, 12:33: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by VarunTYAGi9013 | 21 Oct, 2023, 07:14: PM