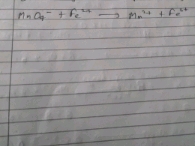CBSE Class 11-science Answered
280 ml of sulphur vapour at ntp weigh 3.2 g. the molecular formula of the sulpher vapour is????
Asked by ayushikas | 22 May, 2011, 10:23: PM
As we know density of a gas = Mass/Volume = 3.2gm / 280mL
Molecular mass = Molar volume x density
As molar volume at N.T.P = 22.4L = 22.4 x 1000mL
So molecular mass = 22.4 x 1000 x 3.2 / 280 = 256
Now molecular formula = n x Empirical formula
n = Molecular mass / Empirical formula mass
Empirical formula mass for sulphur is = 32
So, n = 256 / 32 = 8
Hence molecular formulaof sulphur vapour is = 8 x (S)
= S8
Answered by | 25 May, 2011, 02:53: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by VarunTYAGi9013 | 21 Oct, 2023, 07:14: PM