ICSE Class 10 Answered
 24 and 25 ques
24 and 25 ques
Asked by lovemaan5500 | 26 Nov, 2017, 12:45: PM
Heat gain by water = mw × Cp × (80-30)
Heat Loss by Steam = ms × L + ms ×Cp × (100-80)
whrere mw is mass of water, Cp is sp.heat of water, ms is mass of steam, L is latent Heat of vaporaisation of steam.
Heat gain = Heat loss
mw × Cp × (80-30) = ms × L + ms ×Cp × (100-80)
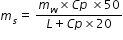
Substitute the values for mw, Cp and L and workout for ms
I got the final answer as ms = 0.756 kg
------------------------------------
Please ask one question per post
Answered by | 27 Nov, 2017, 03:12: PM
Concept Videos
ICSE 10 - Physics
Asked by imunilu786 | 29 Oct, 2023, 02:35: PM
ICSE 10 - Physics
Asked by surendrakumarclw | 20 Mar, 2021, 06:16: PM
ICSE 10 - Physics
Asked by anuradhastudent12 | 27 Feb, 2021, 08:33: PM
ICSE 10 - Physics
Asked by khushalkumarsk | 17 Aug, 2020, 12:37: PM
ICSE 10 - Physics
Asked by abhinabasarkar.abhinaba | 17 Jun, 2020, 09:24: PM
ICSE 10 - Physics
Asked by Mail.nehasharmaa | 20 Feb, 2019, 09:02: AM
ICSE 10 - Physics
Asked by piyush211975 | 15 Feb, 2019, 03:48: PM
ICSE 10 - Physics
Asked by swainanirudha1 | 16 Nov, 2018, 04:04: PM




