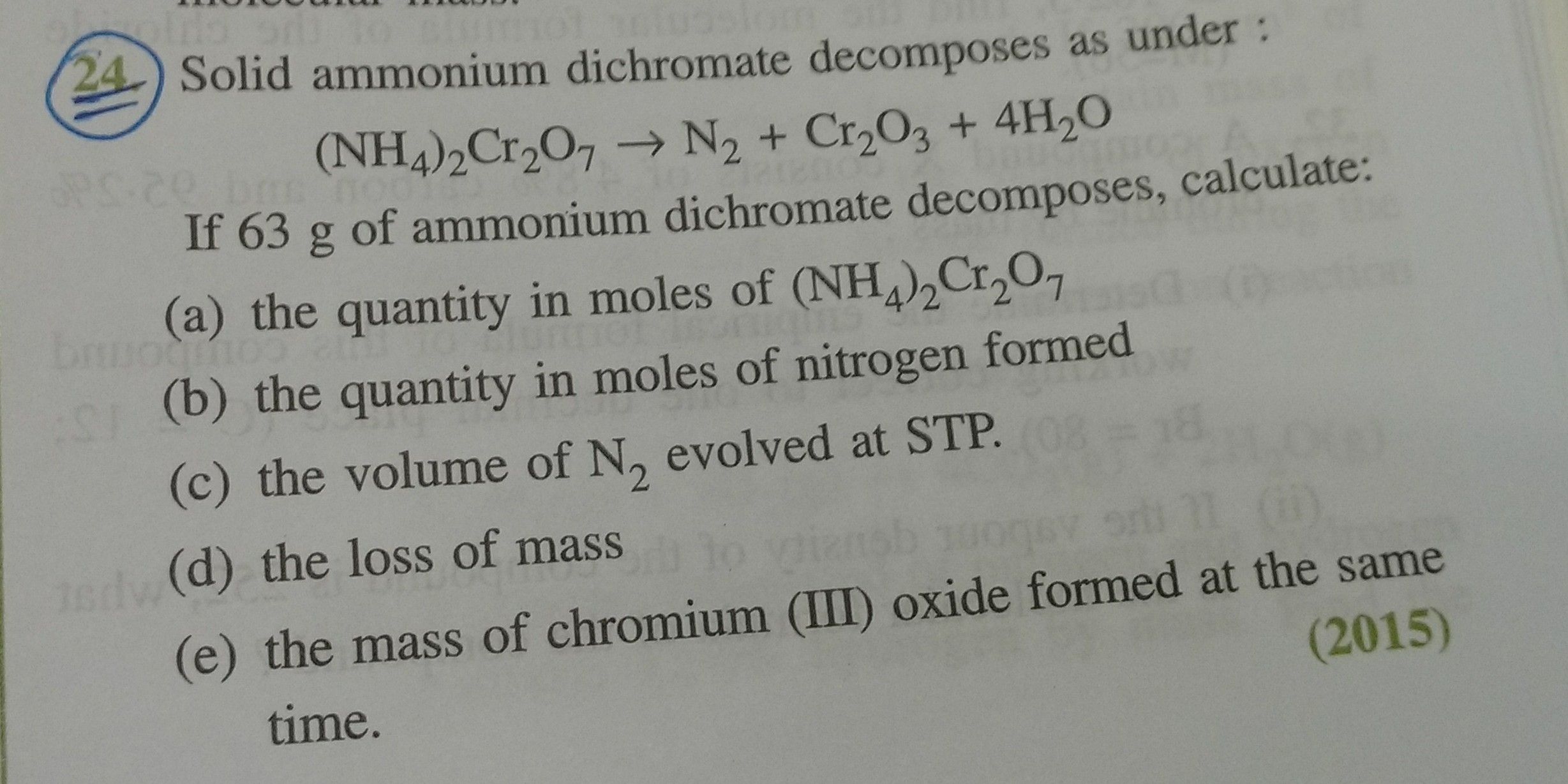ICSE Class 10 Answered
224 lt.of ammonia undergoes catalytical oxidation in presence of Pt to give nitric oxide and water vapour .Calculate thvolume of oxygen required for the reaction.All the volumes measured at room temperature and pressure.
Asked by Sayantany mukhopadhyay | 02 Aug, 2014, 09:23: AM
Reaction:
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(l)
4vol. 5vol. 4vol. 6vol.
Volume of H2O will be negligible as it is in the liquid form at room temperature.
(Note: Gay Lussac’s Law is not applicable to substances in liquid and solid states).
According to Gay Lussac’s Law,
4vols. of NH3 require 5vols. Of oxygen
4 cm3 of NH3 require 5 cm3 Of oxygen
Therefore, 224 cm3 of NH3 require oxygen = 5/4 × 224 = 280 cm3
Hence, volume of oxygen required = 280 cm3
4vols. of NH3 produce 4vols of NO
4 cm3 of NH3 produce 4 cm3 of NO
Therefore 224 cm3 of NH3 produce NO = 4/4 × 224 = 224 cm3
Hence, volume of NO produced = 224 cm3
Answered by Hanisha Vyas | 04 Aug, 2014, 11:56: AM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by maybe.kushagra | 25 Jan, 2024, 03:12: AM
ICSE 10 - Chemistry
Asked by srinu2020.ravipati | 16 Sep, 2020, 03:33: PM
ICSE 10 - Chemistry
Asked by Gurdev71 | 24 Jun, 2020, 12:41: PM
ICSE 10 - Chemistry
Asked by Kanwaranita10 | 16 Feb, 2020, 11:22: AM
ICSE 10 - Chemistry
Asked by aashimegh | 17 Aug, 2019, 02:24: PM
ICSE 10 - Chemistry
Asked by aashimegh | 03 Aug, 2019, 11:50: AM
ICSE 10 - Chemistry
Asked by Shrinivasdangi07 | 21 Mar, 2019, 10:34: PM
ICSE 10 - Chemistry
Asked by johncena9384 | 26 Oct, 2018, 04:08: PM
ICSE 10 - Chemistry
Asked by yajay0441 | 27 Aug, 2018, 03:15: PM