CBSE Class 11-science Answered
1) With the help of the following information, one can find out the hybridisation of carbon in the compound.
sp3 hybridisation involves 4 hybrid orbitals that mean the carbon atom is attached to the four different atoms.
sp2 hybridisation involves 3 hybrid orbitals which mean carbon is attached to three different atoms.
And in the case of sp hybridisation there are two hybrid orbitals are involved hence carbon will be attached to two atoms.
2) Sigma bond: When the axes of orbitals and their axis of joining are the same the covalent bond formed by their overlapping is called a sigma bond.
In other words, the axial or head-on overlapping of two half-filled atomic orbitals forms a sigma bond.
Pi bond: When the axes of orbitals and their axis of joining are perpendicular the bond formed by their overlapping is called a pi bond.
In other words, the sidewise or lateral overlapping of two half-filled atomic orbitals forms a pi bond.








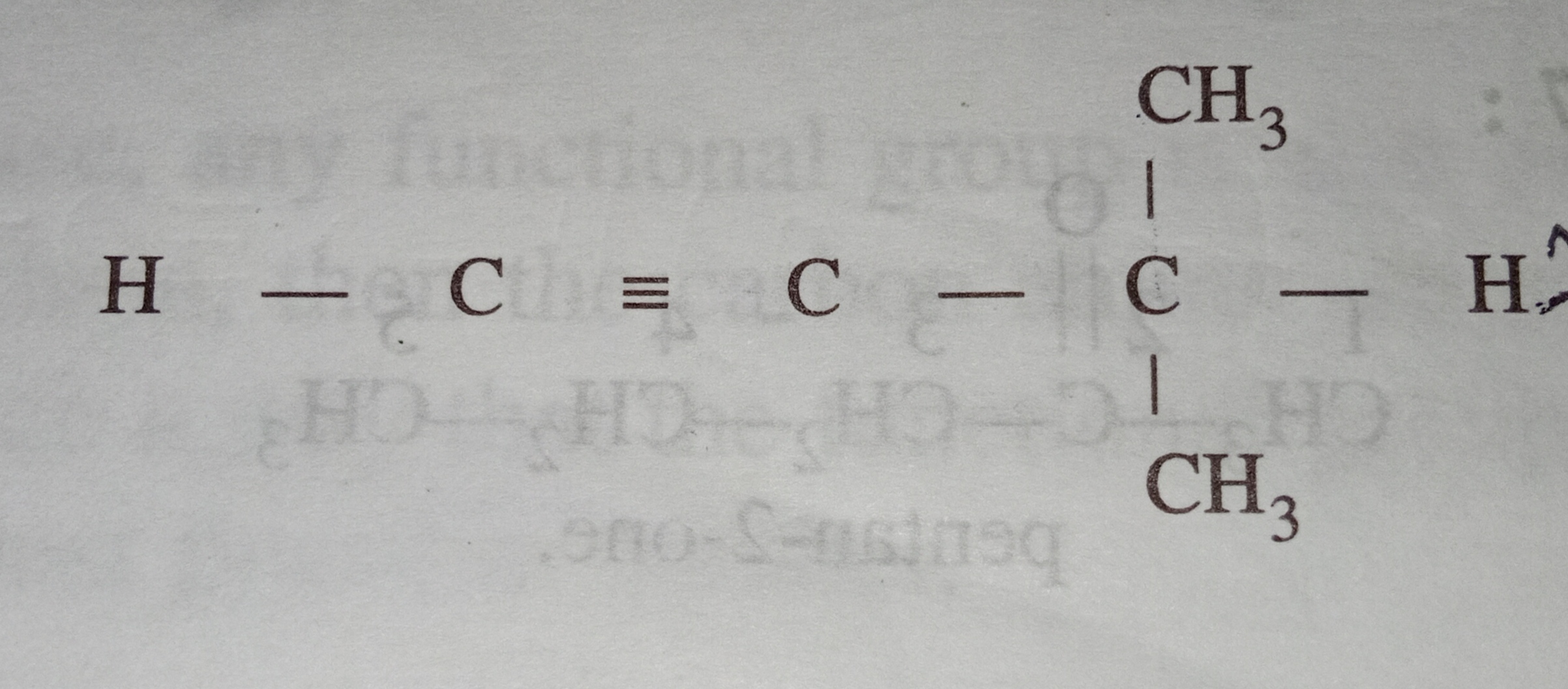
 hybridised ,how will it be classified?
hybridised ,how will it be classified?