JEE Class main Answered
1.102 ques

Asked by lovemaan5500 | 30 Jan, 2019, 12:01: PM
Given:
Mole fraction of benzene = 0.4
Total number of moles in a solution = 1
No. of moles of benzene = 0.4 mol
Weight of benzene = no. of moles × molecular mass
= 0.4 × 78
= 31.2 g
Mole fraction of toluene = 0.6
Moles of toluene = 0.6 mol
Weight of toluene = 0.6 × 92.14
= 55.28 g
Total weight = 31.2 + 55.28
=86.48
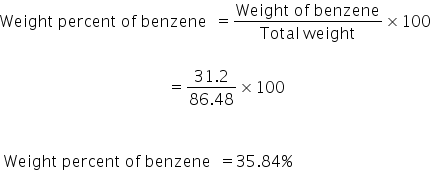
Weight percent of benzene is 35.84%
Answered by Varsha | 30 Jan, 2019, 05:22: PM
JEE main - Chemistry
Asked by amarnathreddyp19 | 29 Mar, 2024, 06:47: AM
JEE main - Chemistry
Asked by atharvamane801 | 14 Jan, 2024, 12:07: PM
JEE main - Chemistry
Asked by bhyogita884 | 12 Jul, 2022, 02:55: AM
JEE main - Chemistry
Asked by abdulraqeeb437 | 16 Jun, 2022, 08:38: PM
JEE main - Chemistry
Asked by akshatmi2005 | 21 May, 2021, 02:23: PM


