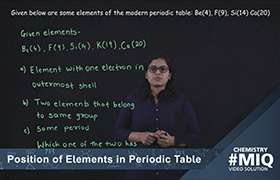
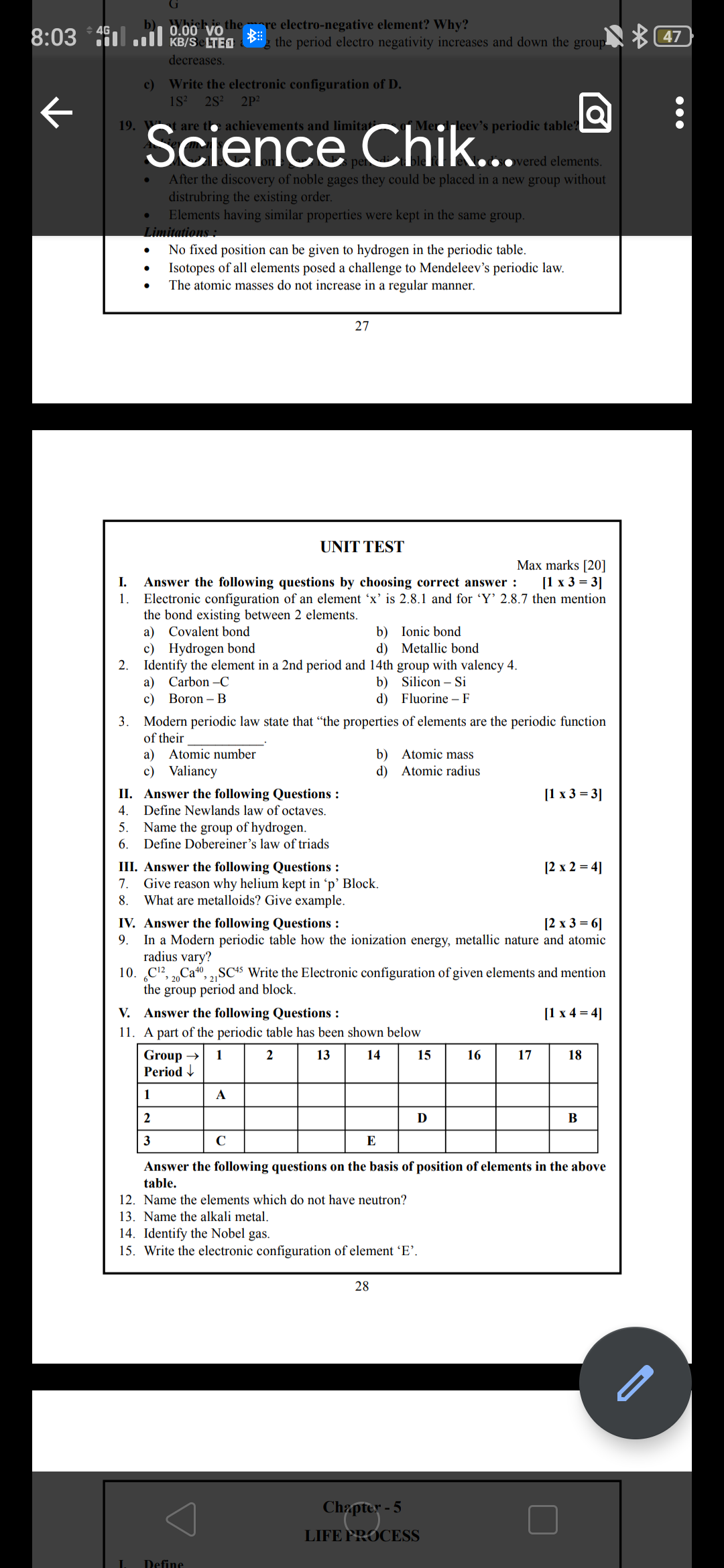
CBSE Class 10 Answered
1. Half-filled and completely filled shells have extra stability associated with them. As a result, it is difficult to remove electron from these stable configurations and ionization energy is high. This may be explained by following examples:
(i) The noble gases have the most stable electronic configurations (ns2 np6) in each period and therefore, have high ionization energies.
(ii)The elements like Be(1s2 2s2) and magnesium (1s2 2s22p6 3s2) have completely filled orbitals and their ionization energies are large.
(iii)The elements like N(1s2 2s2 2px12py1 2pz1) and P (1s2 2s2 2p6 3s2 3px1 3py1 3pz1) have the configurations in which the orbitals of the same subshell are exactly half-filled and are also stable. Hence, they need large energy to remove the electron that is their ionization energies are high.
Thus, the more stable the electronic configuration, the greater is the ionization energy.
2. Shells are divided into subshells, which are further divided into orbitals.
Electrons are present outside the nucleus and revolve around it in shells K, L, M, N etc.
Each shell consists of subshell. K contains only one s subshell. L contains s & p subshell. M contains s, p & d subshell. N contains s, p, d & f subshell.
The s-subshell containing only one orbital can have a maximum of 2 electrons.
The p-subshell containing three orbitals can have a maximum of 6 electrons.
The d-subshell containing five orbitals can have a maximum of 10 electrons.
The f-subshell containing seven orbitals can have a maximum of 14 electrons.
3. The order of filling is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 6f, 5d, 7p.