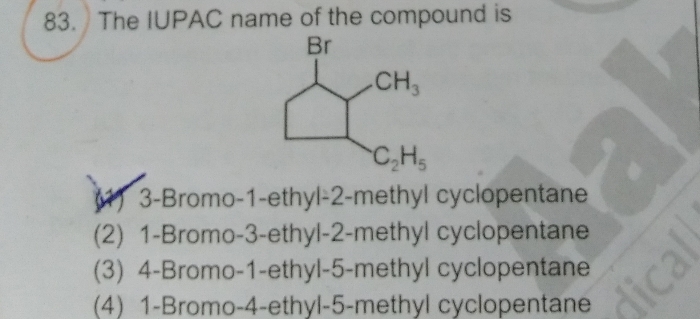
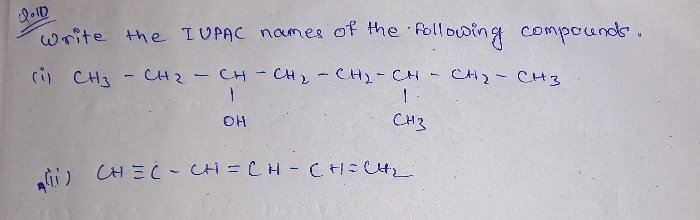
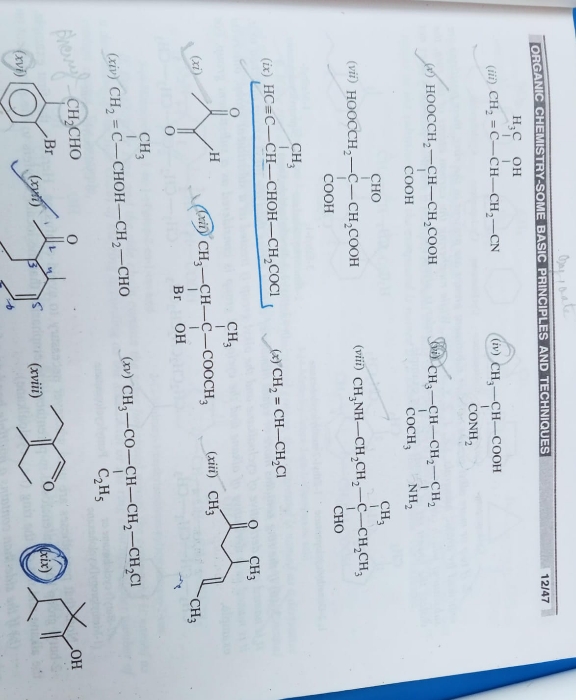
CBSE Class 11-science Answered
0.2325gh of organic compound was analysed for nitrogen by dumas method ,31.7 ml of moist nitrogen gas was collected at 25 C and 7555.8mm pressure. determine the % of nitrogen (aqueous tension (f)=23.8mm at 25 C)
2.0.151g of organic compound containing phosphorous gave 0.214g of magnesium pyrophosphate for the estimation of phosphorus. calculate the % of phosphorus.
Asked by Subiya Khan | 11 Oct, 2013, 10:35: AM
1. To determine the % of nitrogen
2. 263 g of Mg3(PO4)2 contains 31 g of phosphorus.
Therefore, 0.214 g of Mg3(PO4)2 will contain  g of phosphorus.
g of phosphorus.
0.151 g of organic compound contains  g of phosphorus.
g of phosphorus.
Therefore, 100g of compound will contain
Answered by Hanisha Vyas | 15 Oct, 2013, 06:31: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by josephineanto1960 | 28 Mar, 2024, 12:50: PM
CBSE 11-science - Chemistry
Asked by neet2025targetgo | 25 Mar, 2024, 10:13: AM
CBSE 11-science - Chemistry
Asked by ap4450962 | 12 Mar, 2024, 07:35: PM
CBSE 11-science - Chemistry
Asked by pamjat.8888 | 31 Jan, 2024, 11:31: AM
CBSE 11-science - Chemistry
Asked by sahumahesh3973 | 20 Jan, 2024, 06:33: PM
CBSE 11-science - Chemistry
Asked by aswintj2007 | 07 Jan, 2024, 08:53: PM
CBSE 11-science - Chemistry
Asked by dipalisingh0908 | 05 Nov, 2023, 02:24: PM
CBSE 11-science - Chemistry
Asked by badalbehera258369 | 19 Oct, 2023, 02:01: PM
CBSE 11-science - Chemistry
Asked by prakrutikhosla | 16 Sep, 2023, 06:31: PM
CBSE 11-science - Chemistry
Asked by shahintkjnv2016 | 13 Jun, 2022, 07:17: PM