Class 9 FRANK Solutions Chemistry Chapter 1 - Matter and its Composition: Law of Conservation of Mass
Get the best Frank Solutions for ICSE Class 9 Chemistry Chapter 1 Matter and its Composition: Law of Conservation of Mass at TopperLearning. Revise the characteristics of a gas, a solid and a liquid. Study the accurate solutions for objective type questions. The solutions will also help you understand the postulates of the kinetic theory of matter.
Our online education portal’s Chemistry revision materials also include ICSE Class 9 Chemistry Selina solutions, videos and more. If you need assistance in understanding the law of conservation of mass, ask an expert for answers in our ‘UnDoubt’ section.
8
9
Matter and its Composition: Law of Conservation of Mass Exercise 8
Solution 3
Light and sound are not considered to be matter because they neither have mass nor do they occupy space.
Solution 1
Matter is anything around us which occupies space and has mass. Example- Coal, Copper, Water, Oxygen, Kerosene
Key: Matter occupies space and has mass.
Key: Matter occupies space and has mass.
Solution 2
The conditions for something to be called matter are-
(i) It should occupy space.
(ii) It should have mass.
(i) It should occupy space.
(ii) It should have mass.
Matter and its Composition: Law of Conservation of Mass Exercise 9
Solution 11
(a) Solid to liquid state: The conversion of a substance from the solid state to liquid state at a particular temperature is called melting or fusion. The heat energy supplied to the solid is absorbed by its molecules to gain kinetic energy. The kinetic energy increases the rate of vibration of the molecules. The force of attraction thus no longer holds the molecules close together and the solid gets change into liquid.
(b) Gas to liquid: The conversion of a substance from the gaseous state to its liquid state at a particular temperature is called condensation or liquefaction. On cooling, the gas molecules loose their kinetic energy in the form of lost heat and so molecular motion slows down. Decreased molecular motion causes a decrease in intermolecular space. The molecules come very close and the force of attraction between them correspondingly increases and the gas gets change into liquid.
(c) Liquid to gaseous state: The heat energy supplied to the liquid is absorbed by its molecules to gain kinetic energy and therefore the molecules move faster. This increases the intermolecular space. The intermolecular force of attraction decreases and liquid changes into gaseous state.
(d) Liquid to solid state: The conversion of a substance from the liquid state to solid state by cooling is called freezing. On cooling a liquid, the kinetic energy of the molecule is decreased. Due to decreased kinetic energy, the molecules cool down and come closer, thus reducing the intermolecular spaces. The force of attraction between the molecules thus increases. Now, the molecules are no longer in a position to be free or to migrate and liquid changes into a solid.
(b) Gas to liquid: The conversion of a substance from the gaseous state to its liquid state at a particular temperature is called condensation or liquefaction. On cooling, the gas molecules loose their kinetic energy in the form of lost heat and so molecular motion slows down. Decreased molecular motion causes a decrease in intermolecular space. The molecules come very close and the force of attraction between them correspondingly increases and the gas gets change into liquid.
(c) Liquid to gaseous state: The heat energy supplied to the liquid is absorbed by its molecules to gain kinetic energy and therefore the molecules move faster. This increases the intermolecular space. The intermolecular force of attraction decreases and liquid changes into gaseous state.
(d) Liquid to solid state: The conversion of a substance from the liquid state to solid state by cooling is called freezing. On cooling a liquid, the kinetic energy of the molecule is decreased. Due to decreased kinetic energy, the molecules cool down and come closer, thus reducing the intermolecular spaces. The force of attraction between the molecules thus increases. Now, the molecules are no longer in a position to be free or to migrate and liquid changes into a solid.
Solution 4
Solid: (i) The particles are held together by strong intermolecular forces and have minimum intermolecular space.
(ii) Solids have definite mass, shape and volume.
Liquid:(i)The intermolecular forces of liquid molecules are intermediate of molecules of solids and gases while intermolecular spaces are greater than in solids.
(ii) Liquids have definite mass and volume but not definite shape. They take the shape of the container.
Gas: (i) The particles are held together by very weak intermolecular forces while intermolecular spaces are much greater than in solids.
(ii) Gases have definite mass but not definite shape and volume. They take the shape of the container.
(ii) Solids have definite mass, shape and volume.
Liquid:(i)The intermolecular forces of liquid molecules are intermediate of molecules of solids and gases while intermolecular spaces are greater than in solids.
(ii) Liquids have definite mass and volume but not definite shape. They take the shape of the container.
Gas: (i) The particles are held together by very weak intermolecular forces while intermolecular spaces are much greater than in solids.
(ii) Gases have definite mass but not definite shape and volume. They take the shape of the container.
Solution 5
Two reasons for saying that wood is a solid are-
(i) Wood has definite mass and shape.
(ii) Their intermolecular forces are very strong so they cannot flow.
(i) Wood has definite mass and shape.
(ii) Their intermolecular forces are very strong so they cannot flow.
Solution 12
(a) Freezing: The process of changing a liquid into a solid by cooling is called freezing. Freezing means solidification. It occurs at a definite temperature called freezing point.
(b) Evaporation: The phenomenon involving the change of a substance from the liquid state to the gaseous state at room temperature or at any other temperature below its boiling point is called vaporisation or evaporation.
(c) Boiling point: The temperature at which a liquid boils and changes rapidly into a gas at atmospheric pressure is called boiling point of the liquid.
(d) Melting point: The temperature at which a solid substance changes into its liquid state at 1 atmospheric pressure is called the melting point of that substance.
(b) Evaporation: The phenomenon involving the change of a substance from the liquid state to the gaseous state at room temperature or at any other temperature below its boiling point is called vaporisation or evaporation.
(c) Boiling point: The temperature at which a liquid boils and changes rapidly into a gas at atmospheric pressure is called boiling point of the liquid.
(d) Melting point: The temperature at which a solid substance changes into its liquid state at 1 atmospheric pressure is called the melting point of that substance.
Solution 6
The particles of gases are separated from each other by large spaces and intermolecular forces of attraction are the weakest in gases. They have least density. So, they can flow easily. Hence, gases have no fixed shape and volume.
Key: Intermolecular forces of attraction are the weakest in gases.
Key: Intermolecular forces of attraction are the weakest in gases.
Solution 13
(a) Size of naphthalene balls decreases - Sublimation
(b) Drying of wet clothes - Evaporation
(c) Wax melts in the sun - Melting
(d) Formation of clouds - Evaporation and Condensation
(b) Drying of wet clothes - Evaporation
(c) Wax melts in the sun - Melting
(d) Formation of clouds - Evaporation and Condensation
Solution 7
(a) Gases
(b) Solid
(c) Solid
(b) Solid
(c) Solid
Solution 14
Three compounds which are sublimate are-
(i) Camphor
(ii) Naphthalene
(iii) Iodine
(i) Camphor
(ii) Naphthalene
(iii) Iodine
Solution 15
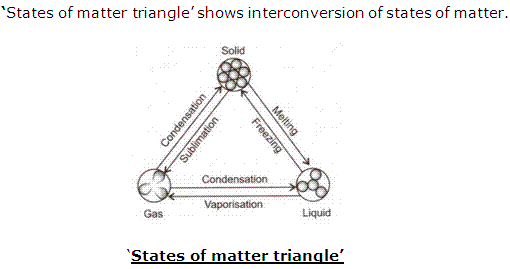
Solution 16
The process by which a liquid slowly converts into vapour state at a temperature below its boiling point is called evaporation.
The heat energy is absorbed by the liquid to convert it into vapour state. So, loss of heat causes cooling.
The heat energy is absorbed by the liquid to convert it into vapour state. So, loss of heat causes cooling.
Solution 8
| Properties | Solids | Liquids | Gases |
| 1.State of packing | The particles are closely packed and their positions are also fixed. | The particles are loosely packed and their positions are not fixed. | The molecules are wide apart and their positions are also not fixed. |
| 2.Energy associated | Particles can vibrate only to and fro about their mean positions. Therefore, they have small kinetic energy due to their motion. | The particles can move about more freely and have considerable kinetic energy due to their motion. | The particles move about freely and have maximum kinetic energy due to their motion. |
| 3.Intermolecular forces | The particles are held together by strong intermolecular forces. | The particles are held together by weak intermolecular forces. | The particles are held together by very weak intermolecular forces. |
| 4.Physical features | Solid has a crystalline structure with both definite size and definite shape. | Liquid does not have a definite shape but has definite volume and can flow from higher to lower level. | Gas has neither definite shape nor a definite volume but can flow and is easily compressible. |
Solution 17
(a) True
(b) True
(c) False
(d) True
(b) True
(c) False
(d) True
Solution 18
"In all physical and chemical changes, the total mass of the reactants is equal to that of the products". So, in other words matter can neither be created nor destroyed.
Solution 19
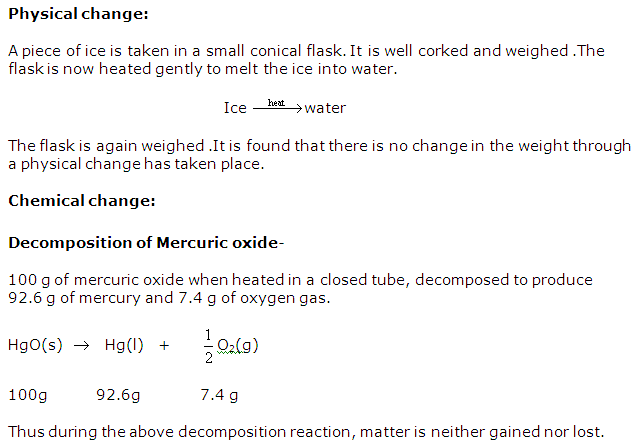
Solution 20
(a) Matter
(b) Solid
(c) Gas
(d) Solid
(e) Gas
(f) Gas
(g) Liquid
(h) Fluid
(i) Melting
(j) Vapourisation
(b) Solid
(c) Gas
(d) Solid
(e) Gas
(f) Gas
(g) Liquid
(h) Fluid
(i) Melting
(j) Vapourisation
Solution 9
(a) gases
(b) strong
(c) sublimation
(d) Condensation
(e) gaseous
(b) strong
(c) sublimation
(d) Condensation
(e) gaseous
Solution 10
The postulates of the kinetic theory of matter-
(a) Composition of Matter: Matter, whether in the solid, liquid or gaseous state, is composed of very small particles which may be molecules, atoms or ions.
(b) Arrangement of Particles: These particles have spaces lying between them and these spaces are referred to as intermolecular spaces or interparticle spaces.
(c) Forces of Attraction: The forces of attraction between the molecules of a given substance are called intermolecular forces. The magnitude of this force depends upon the state of the substance and on the magnitude of the intermolecular spaces. As the intermolecular space increases, the intermolecular force decreases.
(d) Motion of the constituent particles: The particles are always in a state of motion. In solids, they vibrate about their mean positions and in liquids and gases, they move randomly.
(e) The kinetic energy of the particles increases with rise in temperature. As the temperature is increased, the particles undergo motion more vigorously and more randomly.
(a) Composition of Matter: Matter, whether in the solid, liquid or gaseous state, is composed of very small particles which may be molecules, atoms or ions.
(b) Arrangement of Particles: These particles have spaces lying between them and these spaces are referred to as intermolecular spaces or interparticle spaces.
(c) Forces of Attraction: The forces of attraction between the molecules of a given substance are called intermolecular forces. The magnitude of this force depends upon the state of the substance and on the magnitude of the intermolecular spaces. As the intermolecular space increases, the intermolecular force decreases.
(d) Motion of the constituent particles: The particles are always in a state of motion. In solids, they vibrate about their mean positions and in liquids and gases, they move randomly.
(e) The kinetic energy of the particles increases with rise in temperature. As the temperature is increased, the particles undergo motion more vigorously and more randomly.

