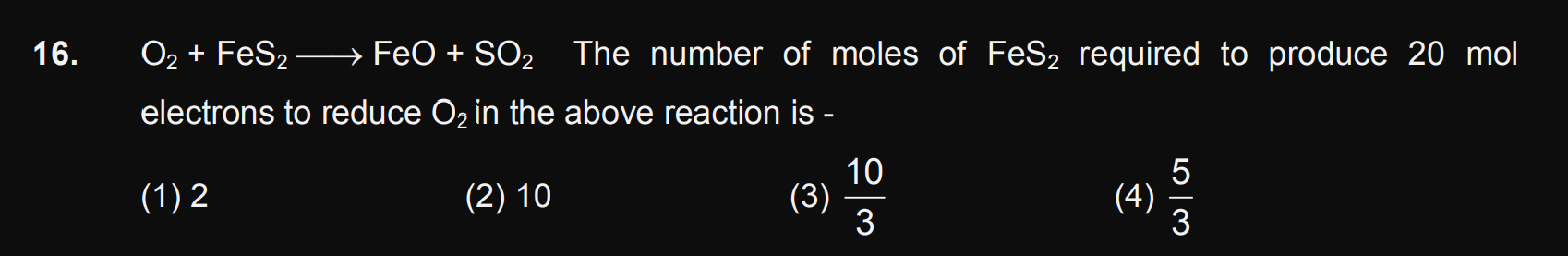Doubts and Solutions
OR
JEE Main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 09:44: PM
JEE Main - Chemistry
Asked by ashwinskrishna2006 | 18 Apr, 2024, 05:37: PM
CBSE IX - Maths
Asked by monika.vns14 | 18 Apr, 2024, 05:15: PM
CBSE X - Physics
Asked by agankitgupta938 | 18 Apr, 2024, 04:29: PM
CBSE XI Science - Maths
Asked by sampabarman328 | 18 Apr, 2024, 01:20: PM
NEET NEET - Physics
Asked by praveenpriya000079 | 18 Apr, 2024, 07:24: AM
CBSE XII Science - Chemistry
Asked by hannamaryphilip | 17 Apr, 2024, 11:20: PM