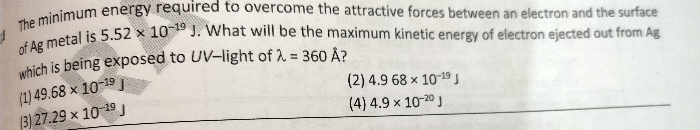Doubts and Solutions
OR
ICSE IX - Maths
Asked by jatinderkaur19852008 | 25 Apr, 2024, 06:25: PM
NEET NEET - Physics
Asked by ramanjaneyuluoguru | 25 Apr, 2024, 04:18: PM
CBSE X - Maths
Asked by raopaidi | 25 Apr, 2024, 04:06: PM
CBSE XII Science - Maths
Asked by leelasri11663 | 25 Apr, 2024, 11:34: AM
CBSE XI Science - Chemistry
Asked by saranyachakraborty2007 | 25 Apr, 2024, 05:23: AM
CBSE XII Science - Maths
Asked by aishaazmata | 24 Apr, 2024, 08:48: PM
ICSE VIII - History and Civics
Asked by khatunrafika276 | 24 Apr, 2024, 06:57: PM