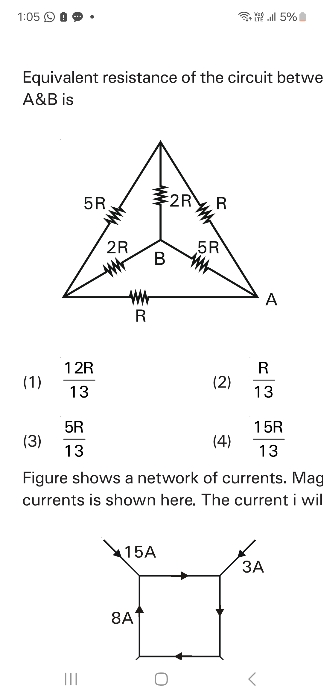
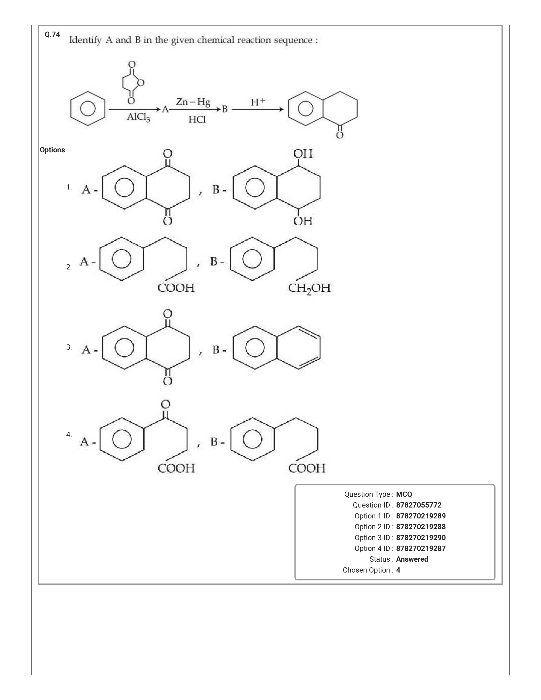
Doubts and Solutions
OR
CBSE VII - Maths
Asked by shouryavijay0020012 | 17 Apr, 2024, 03:45: PM
CBSE XI Commerce - Accountancy
Asked by hydrogamerz.piyush | 17 Apr, 2024, 02:18: PM
CBSE VIII - Maths
Asked by japjigrewal54 | 17 Apr, 2024, 01:54: PM
CBSE XI Science - Maths
Asked by tahikpreet0001 | 17 Apr, 2024, 06:41: AM
NEET NEET - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
CBSE VI - Grammar
Asked by beherajyoti253 | 16 Apr, 2024, 08:11: PM
CBSE XII Science - Maths
Asked by mahammadsharifdakhani67 | 16 Apr, 2024, 07:43: PM